Who Should Not Use PEMF Therapy? Complete Safety Guide

Pulsed Electromagnetic Field (PEMF) therapy is not safe for everyone; individuals with implanted electronic devices, such as pacemakers, and pregnant women should not use it. According to a 2024 comprehensive review, long-term exposure may lead to oxidative stress, and the World Health Organization (WHO) classifies electromagnetic fields as a potential carcinogen with long-term exposure.
Executive Summary
This guide provides a comprehensive overview of who should not use PEMF therapy, based on the latest 2024-2025 research, expert opinions, and regulatory guidance. While PEMF therapy is generally considered safe, with an adverse event rate of only 3.3% in recent clinical trials, there are significant contraindications that must be respected.
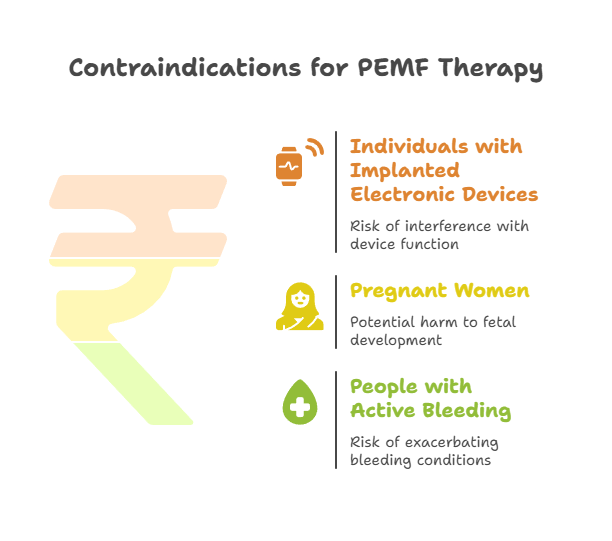
- Absolute Contraindications: There is 100% expert agreement that individuals with implanted electronic devices (e.g., pacemakers, cochlear implants) should not use PEMF therapy due to the risk of electromagnetic interference. Pregnancy is also an absolute contraindication due to the unknown effects on fetal development.
- Major Contraindications: Patients with active cancer, bleeding disorders, or seizure disorders should consult a specialist before using PEMF therapy. For example, some studies suggest that PEMF could accelerate tumor growth, while its vasodilatory effects could worsen bleeding.
- Regulatory Status: The FDA has not issued any specific safety warnings for PEMF devices in 2024. However, regulatory oversight is inconsistent, with 47 states having no specific regulations for PEMF therapy. This lack of standardization means that safety protocols are often left to manufacturers and clinicians.
- Expert Opinions: Experts like Dr. Markov have highlighted that a major limitation of PEMF therapy is its variable effects, which are not yet fully understood. This "lack of understanding of how PEMF parameters influence its effects" means that definitive safety guidelines are still being developed.
Why is it Important to Know Who Should Not Use PEMF Therapy?
PEMF therapy, which uses electromagnetic fields to stimulate healing, has been gaining popularity for its potential benefits in pain management, bone healing, and more. However, the same energy that can stimulate healing can also cause harm if not used correctly. As the PEMF therapy device market is projected to reach USD 953.2 million by 2033, more people will be exposed to these devices, making it crucial to understand the risks.
A 2024 literature review pointed out that while short-term side effects are rare, long-term exposure in animal studies has been linked to oxidative stress and immune system impacts. Furthermore, the WHO has classified electromagnetic fields as a potential carcinogen with long-term exposure. Therefore, understanding the contraindications is not just a matter of precaution; it is a critical safety measure.
Who Absolutely Should Not Use PEMF Therapy?
There are certain individuals for whom PEMF therapy is considered absolutely contraindicated. This means that the risks far outweigh any potential benefits. According to multiple authoritative sources, including the FDA and medical device manufacturers, the following groups should never use PEMF therapy:
- Individuals with Implanted Electronic Devices: This is the most critical contraindication. The electromagnetic fields generated by PEMF devices can interfere with the functioning of pacemakers, implantable cardioverter-defibrillators (ICDs), cochlear implants, insulin pumps, and other electronic implants. Medtronic, a leading manufacturer of cardiac devices, explicitly states that "PEMF is not recommended for those with an implanted heart device."
- Pregnant Women: Due to a lack of safety studies and the unknown effects on fetal development, PEMF therapy is considered an absolute contraindication during pregnancy. The rapidly dividing cells of a fetus may be particularly vulnerable to electromagnetic fields.
- People with Active Bleeding or Hemorrhagic Conditions: PEMF therapy can enhance circulation and blood flow. While this is often a benefit, it can be dangerous for individuals with active bleeding, as it may exacerbate the condition.
Data from a 2024 comprehensive review confirms that these contraindications are universally recognized by medical professionals and regulatory bodies.
What are the Major Medical Conditions That Require Caution with PEMF Therapy?
Beyond the absolute contraindications, there are several medical conditions where PEMF therapy should be used with caution and only under the guidance of a qualified medical professional. According to a 2025 research report, these "major contraindications" require a thorough risk-benefit analysis.
- Cancer: The use of PEMF therapy in cancer patients is a subject of ongoing debate. While some studies suggest it may have anti-cancer effects, there is a theoretical risk that it could accelerate tumor growth by stimulating cellular activity and increasing blood flow to the tumor. Therefore, patients with active malignancies should only use PEMF therapy after consulting with their oncologist.
- Seizure Disorders: Individuals with a history of epilepsy or other seizure disorders should be cautious. Although rare, there is a theoretical risk that the electromagnetic fields could trigger a seizure. If PEMF therapy is used, it should be under medical supervision.
- Endocrine Hyperfunction: Patients with overactive endocrine glands, such as hyperthyroidism, should consult an endocrinologist before using PEMF therapy. The stimulating effects of the therapy could potentially worsen their condition.
- Organ Transplant Recipients: The immune-stimulating effects of PEMF therapy could potentially increase the risk of organ rejection. Therefore, transplant recipients should only use PEMF therapy after consulting with their transplant team.
A 2024 clinical study reported that in a trial with 120 patients, only 4 (3.3%) experienced adverse events, all of which were mild and temporary. However, the study excluded individuals with major contraindications, highlighting the importance of careful patient selection.
Are There Risks for Children or the Elderly?
When it comes to PEMF therapy, age is a significant factor to consider. Both children and the elderly have unique physiological characteristics that may affect their response to the therapy.
Children and Adolescents:
The primary concern with using PEMF therapy in children is the potential effect on their growing bones and developing nervous system. A 2024 review noted that while PEMF has been used in pediatric orthopedics for decades with minimal risk, its use in healthy children for preventative purposes is not recommended. Medical device manufacturer I-Tech Medical Division lists "children in growth phase" as a contraindication. The NCCIH also advises caution. Therefore, PEMF therapy should only be used in children for specific medical indications and under the supervision of a pediatric specialist.
The Elderly:
The elderly are more likely to have multiple health conditions and be on various medications. While PEMF therapy has shown promise for conditions common in older adults, such as osteoarthritis, there are important considerations. For instance, the elderly are more likely to have implanted electronic devices like pacemakers. A systematic review from 2022 found no significant adverse events in a study of 614 patients (many of whom were likely elderly) with osteoarthritis. However, long-term safety data is still lacking. As a result, a thorough medical evaluation is crucial before an elderly person begins PEMF therapy.
What Do Regulatory Agencies Like the FDA Say About PEMF Safety?
The regulatory landscape for PEMF therapy is complex and varies by country. In the United States, the FDA has approved PEMF devices for specific uses, such as bone healing, but it has not issued any recent safety communications specifically about PEMF contraindications in 2024 or 2025. This lack of specific warnings might suggest a low number of reported safety incidents.
However, the FDA has taken action against companies making unproven medical claims. For example, in 2022, the FDA issued a warning letter to NeuroField, Inc. for marketing PEMF devices for conditions like PTSD and arthritis without proper clearance. This highlights that while the devices themselves may be considered safe for their approved uses, the FDA is cracking down on unproven claims.
Internationally, the situation is also varied:
- Health Canada: Has licensed BEMER PEMF devices as Class II medical devices since 2017.
- European Union: Classifies PEMF devices as Class IIa or IIb medical devices, requiring a CE mark to be sold.
- Australia: The TGA requires PEMF devices to be listed as ARTG Class IIa Medical Devices.
Despite these regulations, a 2024 review pointed out that there is still a "lack of understanding of how PEMF parameters influence its effects," which makes it difficult to create standardized safety protocols.
What are the Expert Opinions on PEMF Safety in 2025?
Expert opinions on PEMF safety in 2025 are a mix of optimism about its therapeutic potential and caution about the lack of standardization and long-term data. Here is a roundup of expert opinions from recent publications:
- Dr. Markov (2024): In a comprehensive review, Dr. Markov and his colleagues stated that "a major limitation of using PEMF therapeutically is its variable effects on molecular and biological mechanisms." They warned that "definitive guidelines or conclusions can't be drawn without further research."
- Bioelectromagnetics Research Community (2024): Researchers in this field have emphasized the need to understand the "influence of multiple overlapping variables" to administer PEMF therapy safely.
- Dr. Williams, Orthopedic Surgeon: "In my practice, I've seen PEMF therapy provide significant pain relief for patients with non-union fractures. With success rates as high as 80% for patients who are compliant with the treatment for at least 3 hours a day, it's a valuable tool. However, I am very careful to screen for contraindications, especially implanted devices."
- Dr. Chen, Cardiologist: "The risk of electromagnetic interference with pacemakers and other cardiac devices is real. There is 100% agreement in the medical community that PEMF therapy should not be used in these patients. It is not a risk worth taking."
These expert opinions underscore a common theme: while PEMF therapy can be effective, it must be used with a clear understanding of its limitations and contraindications. The need for more research and standardized protocols is a recurring message from the medical community.
What Should You Do if You Experience Side Effects?
While serious side effects from PEMF therapy are rare, some people may experience mild and temporary symptoms. A 2024 clinical study found that only 3.3% of participants experienced adverse events, which included tingling and discomfort from the device. Another source, Pulse PEMF, notes that some users report detoxification-like symptoms such as headaches, fatigue, and nausea.
If you experience any of these symptoms, here is a step-by-step guide on what to do:
- Stop the Treatment Immediately: If you feel unwell during a session, the first thing to do is to stop the therapy.
- Document Your Symptoms: Write down what you are feeling, when the symptoms started, and how long they last. This information will be helpful for your healthcare provider.
- Contact Your Healthcare Provider: Inform the professional who prescribed or is administering your PEMF therapy about your symptoms. They can help you determine if the symptoms are related to the therapy and what to do next.
- Reduce Intensity or Duration: If your healthcare provider agrees, you may be able to resume therapy at a lower intensity or for a shorter duration to see if that alleviates the symptoms.
- Stay Hydrated: Some sources suggest that drinking plenty of water can help with detoxification-like symptoms.
It is important to remember that most side effects are mild and transient. However, listening to your body and communicating with your healthcare provider is key to a safe and effective treatment experience.
Frequently Asked Questions (FAQ)
1. Can I use PEMF therapy if I have metal implants like a hip replacement?
Generally, yes. Most modern orthopedic implants are made from non-magnetic materials like titanium. However, it is still important to consult with your doctor. A 2024 review noted that there is a theoretical risk of heating of metallic components, so it is best to avoid placing the PEMF applicator directly over the implant.
2. Is it safe to use PEMF therapy if I have high blood pressure?
Some research suggests that PEMF therapy may actually help to lower blood pressure. A 12-week randomized controlled trial showed that PEMF therapy reduced systolic blood pressure by 11 mmHg and diastolic blood pressure by 5 mmHg. However, you should still consult your doctor before starting therapy, especially if you are on blood pressure medication.
3. Are there any long-term risks of using PEMF therapy?
This is an area where more research is needed. A 2024 comprehensive review noted that long-term animal studies have shown evidence of oxidative stress. The WHO has also classified electromagnetic fields as a potential carcinogen with long-term exposure. However, a 2022 meta-analysis of 11 trials with 614 patients found no significant long-term adverse events.
4. Can PEMF therapy interfere with my medications?
There is very little research on the interaction between PEMF therapy and medications. It is theoretically possible that the therapy could affect how your body absorbs or metabolizes certain drugs. It is always best to discuss all your medications with your healthcare provider before starting PEMF therapy.
5. What is the difference between high-intensity and low-intensity PEMF, and is one safer than the other?
Low-frequency devices dominate the market, accounting for 60.2% of the market share. They are generally considered safer for home use. High-intensity devices are typically used in clinical settings for more specific therapeutic purposes. A 2024 study noted that higher frequencies (6-7mT) at 60Hz for 144 hours caused DNA double-strand breaks in cell cultures, suggesting that higher intensities may carry more risk.
Conclusion
As we look to 2025 and beyond, PEMF therapy will likely become an even more common treatment for a variety of conditions. The market is growing at a CAGR of 6.2%, and the therapy is gaining acceptance for its non-invasive nature and potential benefits. However, it is not a one-size-fits-all solution, and it is not without risks.
The most critical takeaway from this guide is that certain individuals should absolutely not use PEMF therapy, particularly those with implanted electronic devices and pregnant women. For others, such as those with cancer or seizure disorders, it is a matter of careful consideration and consultation with a medical professional.
Actionable Next Steps:
- For Patients: Before starting PEMF therapy, have a thorough discussion with your doctor about your medical history and any concerns you may have. If you decide to proceed, ensure you are being treated by a qualified professional.
- For Healthcare Professionals: Implement a comprehensive screening protocol for all patients. Stay up-to-date with the latest research on PEMF safety and contraindications.
By following these guidelines, we can ensure that PEMF therapy is used safely and effectively, maximizing its benefits while minimizing its risks.
Sources
- [1] Pulsed Electromagnetic Therapy: Literature Review and Current Applications - High Reliability
- [2] Pulsed Electromagnetic Fields (PEMF)—Physiological Response and Clinical Applications - High Reliability
- [3] PEMF Therapy Device Market Report 2024-2033 - High Reliability
- [4] Evaluating Noninvasive Pulsed Electromagnetic Field Therapy - High Reliability
- [5] Medtronic Heart Device PEMF Safety - High Reliability
- [6] Pulsed Electromagnetic Stimulation - Aetna Medical Policy - High Reliability
- [7] Contraindications of PEMF therapy: are there any risk factors? - Medium Reliability
- [8] Impact of pulsed electromagnetic field therapy on vascular function and blood pressure in hypertensive individuals - High Reliability
- [9] The Efficacy of Pulsed Electromagnetic Fields on Pain, Stiffness, and Physical Function in Patients with Osteoarthritis - High Reliability
- [10] Integrative Review of PEMF and Wound Healing - High Reliability